https://www.pmda.go.jp/english/
•Pharmaceuticals and Medical Devices Agency (PMDA).
•PMDA is a regulatory authority of Japan that collaborates with the Ministry of Health, Labour and Welfare (MHLW).
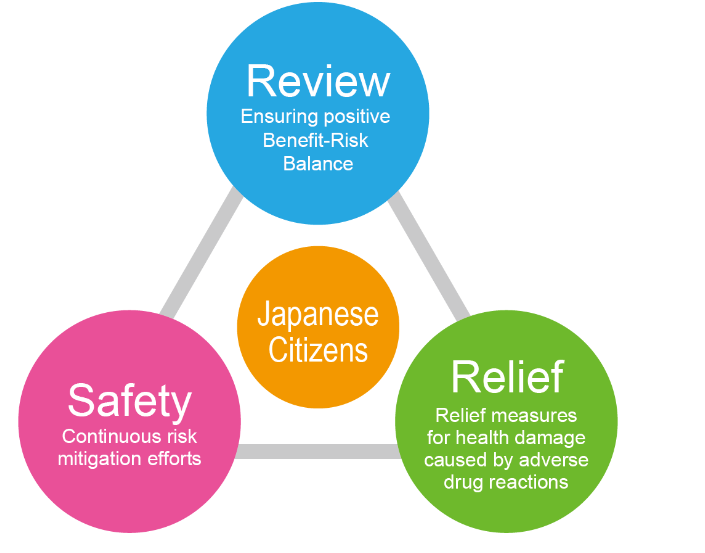
•The PMDA’s obligation is to protect public health by ensuring the safety, efficacy and quality of pharmaceuticals and medical devices.
•The PMDA conducts scientific reviews of marketing authorization applications for pharmaceuticals and medical devices, and monitors their post-marketing safety. Additionally, the PMDA is responsible for providing relief and compensation to patients who experience adverse drug reactions or infections caused by pharmaceuticals or biological products.